Which of the Following Is a Carboxylic Acid Apex
What is the name of the linear hydrocarbon with the molecular formula C H18. The formula used for a carboxylic acid is RCOOH where R refers to the rest of the molecule.

Which Shows The General Structure Of A Carboxylic Acid Brainly Com
Which of the following compound is a carboxylic acid.

. Carboxylic acids are derivatives of hydrocarbons in which one or more of the hydrogen atoms in the hydrocarbon have been. The R group. Structure of Acid Natural Source Common Name.
A CH3CH2NH2 b CH3COOH C CH3CONH2 d CH3CN O a. The name carboxyl derived from the first four letters of carbonyl and the last four letters of hydroxyl. 2 3 and 3.
The p K a values of four carboxylic acids are 4. A carboxylic acid is an organic compound that contains a carboxyl group. The two main kinds of fatty acids are unsaturated and saturated fatty acids.
A carboxylic acid is an organic acid that contains a carboxyl group COOH attached to an R-group. The carboxyl COOH group is so-named because of the carbonyl group CO and hydroxyl group. An acid is any compound that donates a hydrogen ion H also called a proton to another compound termed a base.
Carboxylic acid is composed of two functional groups carbonyl group -CO- and hydroxyl group-OH. CH3CONH2 All of the following are conformations of glucose with the exception of Chair b boat 0 Fischer d Haworth projection a boat b. Here the R group is a side group that can contain hydrogen andor carbon and other atoms.
Acidity of Carboxylic Acids. The most basic way to show a carboxylic acid is R---. Carboxylic acid any of a class of organic compounds in which a carbon C atom is bonded to an oxygen O atom by a double bond and to a hydroxyl group OH by a single bond.
Important examples include the amino acids and fatty acidsDeprotonation of a carboxylic acid gives a carboxylate anion. Greek Letter System for Common Carboxylic Acids. The test which is used for the.
The p K a value of strongest carboxylic acid among them is. Chemistry questions and answers. Which of the following compounds is a ketone.
Chapter 17 Organic Chemistry Carboxylic Acids study guide by Axelknows includes 54 questions covering vocabulary terms and more. A fatty acid refers to a lengthened hydrocarbon chain which comprises a carboxylic group. Carboxylic acids are hydrocarbon compounds in which a carboxyl group has substituted one or more of the hydrogen atoms in the hydrocarbon.
Read more Carboxylic Acids MCQs. The common names of some basic carboxylic acids are derived from Latin names that indicate the first original natural source of the carboxylic acid. Carboxylic acids are organic acids characterized by a carboxyl -COOH functional group.
The organic acid that does not has COOH group is A. Definition of carboxylic acid Organic compounds containing carboxyl functional group -COOH are called carboxylic acid. Methanoic acid HCOOH ethanoic acid CH3COOH propanoic acid C2H5COOH and butanoic acid C3H7COOH are the first four carboxyl acids derived from alkanes.
The acid with the carboxyl group attached directly to a benzene ring is called benzoic acid C 6 H 5 COOH. The structural formula of a carboxylic acid is RCOOH as shown in the following illustration. In case if a fatty acid comprises more than one double bond than it is termed as polyunsaturated fatty acid.
Carboxylic acids contain at least one carboxyl group. When we compare these values with those of comparable alcohols such as ethanol pK a 16 and 2-methyl-2-propanol pK a 19 it is clear that carboxylic acids are stronger acids by over ten powers of ten. A fourth bond links the carbon atom to a hydrogen H atom or to some other univalent combining group.
In substituted benzenecarboxylic acids the ring carbon atom bearing the carboxylic group is always the C 1. The simplest aromatic carboxylic acid is called benzenecarboxylic acid. Quizlet flashcards activities and.
The general formula for carboxylic acids is R COOH where R refers to the rest of the molecule. The acid part of an amino acid is the functional group carboxylic acid COOHThe amine group -NH2 is acidic. The general molecular formula for carboxylic acids is C n H 2n1 COOH.
The general formula of a carboxylic acid is RCOOH or RCO 2 H with R referring to the alkyl alkenyl aryl or other groupCarboxylic acids occur widely. The pK a s of some typical carboxylic acids are listed in the following table. The naming of these compounds is governed by IUPAC nomenclature which ensures systematic and consistent naming of chemicalsNumerous organic compounds have other common names often originating in historical source material thereof.
An unsaturated fatty acid comprises a double bond within the fatty acid chain. Which of the following compounds is a carboxylic acid. Acidity of carboxylic acids is generally higher compared to simple phenols as they react with weak bases like carbonates and bicarbonates to liberate carbon dioxide gas.
The most important property of carboxylic acids and the one that is responsible for naming them such is their acidity. Carboxylic acids contain an OH group and a double bonded Oxygen atom to one carbon which is attached to any hydrocarbon. Salts and esters of carboxylic acids are called carboxylates.
Carboxylic acids are widely used and they include amino acids and acetic acid. Ants Formica Formic acid Vinegar Acetum Acetic acid Basic Fat Propio Propionic acid Rancid butter Butyrum Butyric acid Present in aValerian herb Valeric acid Goat Caper. Idk what that is but the answer is Carboxl functional group.
Carboxylic acids with two or more carboxyl groups attached are called dicarborxylic acids tricarboxylic acids etc. The general formula of carboxylic acid is. Which one of the following acids is present in lemon juice.
Carboxylic acids do this much more readily than most other classes of organic compounds so they are said to be stronger acids even though. The four acids illustrated here are formic acid a acetic acid b propionic acid c and butyric acid d. Carboxylic acids occur widely and include the amino acids and acetic acid.

Functional Groups Ck 12 Foundation

Which Of The Following Is A Carboxylic Acid Brainly Com
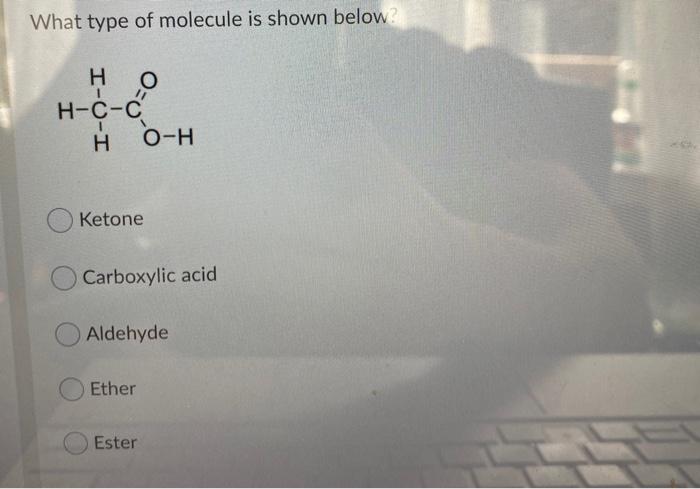
Solved What Type Of Molecule Is Shown Below O H C C O H Chegg Com
Comments
Post a Comment